(→Microfluidics) |
(→Dielectrophoretic cytometry) |
||
| (10 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | = | + | =Lab-on-chip systems= |
| − | + | ||
| − | + | ||
[[File:master.jpg|thumb|right|alt=Silicon master for the PDMS soft-lithography.|A soft-lithography silicon master used for fabrication of the microfluidic devices at Georgiev Lab.]] | [[File:master.jpg|thumb|right|alt=Silicon master for the PDMS soft-lithography.|A soft-lithography silicon master used for fabrication of the microfluidic devices at Georgiev Lab.]] | ||
| − | == | + | Lab-on-chip systems integrate one or more lab functions on a chip of just couple centimeters squared. Such systems are able to work with extremely low volumes of liquid samples, can significantly decrease costs of individual operations and decrease requirements on additional hardware. Hence they enable for instance implementation of affordable diagnostic tools for cancer or development of tools enabling selection of optimal therapy increasing treatment efficiency and its personalization for individual patients. |
| + | |||
| + | Our research focuses on applications of the electric field in lab-on-chip systems in the form of dielectrophoretic effect based on polarization effect in the electric field. Dielectrophoretic effect may be used for measurement of cell properties on population level, their selective contactless manipulation or immobilization. At the same time, it may be combined with other electric field-based methods such as the electro-rotation, the impedance spectroscopy and the electro-poration, and with other technologies such as microscopy and optical data analysis. | ||
| + | |||
| + | == Dielectrophoretic cytometry == | ||
| + | |||
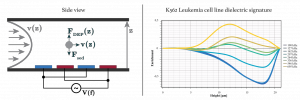
| + | [[File:DDEP_cyto.png|thumb|right|alt=Illustration of 2DEP cytometr.| Illustration of the 2DEP cytometry (left) and the dielectric signature of K562 leukemia cell line measured using 2DEP cytometry.]] | ||
| + | |||
| + | Cytometry in general, is the measurement of characteristics of cells. Flow cytometry quantifies light scattered from cells and their fluorescence while passing through a laser beam in order to analyze cells size and volume, expression of cell surface and intracellular molecules, differentiate between cell types in heterogenous populations, and identify cells with specific structural and dynamic properties. Where conventional flow cytometry requires staining or labelling with antibodies, DEP is able to differentiate between cell types and cells in certain cell states by measuring differences in their Dielectric (DE) | ||
| + | signatures. | ||
| + | |||
| + | DE properties that dictate cell interactions with external electric fields are strongly linked to the cell structural properties, and the cell internal state. Experimental results show DE properties present valuable biomarkers for various cellular events ranging from cell growth, proliferation, response to drug molecules, apoptosis, and cell death. These processes are often mapped to different parts of the cell and thereby manifest as DE changes in different parts of the frequency spectrum. Hence spectral analysis of DE properties can uncover specific physiological changes and provide insight into cellular processes that are otherwise difficult to measure. | ||
| − | [[File: | + | [[File:DDEP_device.jpg|thumb|left|alt=2DEP cytometry chip.| Photo of the actual 2DEP cytometry device.]] |
| − | + | In Georgiev lab, we developed a novel force equilibrium method called Distributed Dielectrophoretic Cytometry (2DEP Cytometry). It uses a DEP-induced vertical translation of live cells in conjunction with Particle Image Velocimetry (PIV) in order to measure probabilistic distribution of live cell DE signatures on an entire cell population. The method is integrated in a micro-fluidic device. It is less sensitive to misalignment of microchannel to electrode topologies enabling PDMS soft lithography to be used for the device fabrication. The bottom of the micro-fluidic channel is lined with an Interdigitated (ID) electrode array. Cells passing through the micro-channel are acted on by sedimentation forces, while DEP forces either oppose sedimentation, support sedimentation, or neither, depending on the DE signatures of the cells. The heights at which cells stabilize correspond to their DE signature and are measured indirectly using PIV. The presented method enables simultaneous and high-throughput collection of hundreds of single-cell responses in a single frame. In addition, PIV may be further integrated with fluorescence measurements yielding correlations between DE signatures and intracellular processes. | |
| − | + | == Dielectrophoretic cell focusing == | |
| − | Dielectrophoretic cell focusing microfluidic device developed at Georgiev Lab. The presence of an electric field tuned to a specific frequency allows the cells to be focused by negative dielectric forces to the center of the channel. | + | Dielectrophoretic cell focusing microfluidic device developed at Georgiev Lab. The presence of an electric field tuned to a specific frequency allows the cells to be focused by negative dielectric forces to the center part of the channel. |
<mediaplayer width='550' height='300'>http://www.ccy.zcu.cz/movies/DEP_Focusing.mp4</mediaplayer> | <mediaplayer width='550' height='300'>http://www.ccy.zcu.cz/movies/DEP_Focusing.mp4</mediaplayer> | ||
Latest revision as of 12:57, 12 May 2017
Lab-on-chip systems
Lab-on-chip systems integrate one or more lab functions on a chip of just couple centimeters squared. Such systems are able to work with extremely low volumes of liquid samples, can significantly decrease costs of individual operations and decrease requirements on additional hardware. Hence they enable for instance implementation of affordable diagnostic tools for cancer or development of tools enabling selection of optimal therapy increasing treatment efficiency and its personalization for individual patients.
Our research focuses on applications of the electric field in lab-on-chip systems in the form of dielectrophoretic effect based on polarization effect in the electric field. Dielectrophoretic effect may be used for measurement of cell properties on population level, their selective contactless manipulation or immobilization. At the same time, it may be combined with other electric field-based methods such as the electro-rotation, the impedance spectroscopy and the electro-poration, and with other technologies such as microscopy and optical data analysis.
Dielectrophoretic cytometry
Cytometry in general, is the measurement of characteristics of cells. Flow cytometry quantifies light scattered from cells and their fluorescence while passing through a laser beam in order to analyze cells size and volume, expression of cell surface and intracellular molecules, differentiate between cell types in heterogenous populations, and identify cells with specific structural and dynamic properties. Where conventional flow cytometry requires staining or labelling with antibodies, DEP is able to differentiate between cell types and cells in certain cell states by measuring differences in their Dielectric (DE) signatures.
DE properties that dictate cell interactions with external electric fields are strongly linked to the cell structural properties, and the cell internal state. Experimental results show DE properties present valuable biomarkers for various cellular events ranging from cell growth, proliferation, response to drug molecules, apoptosis, and cell death. These processes are often mapped to different parts of the cell and thereby manifest as DE changes in different parts of the frequency spectrum. Hence spectral analysis of DE properties can uncover specific physiological changes and provide insight into cellular processes that are otherwise difficult to measure.
In Georgiev lab, we developed a novel force equilibrium method called Distributed Dielectrophoretic Cytometry (2DEP Cytometry). It uses a DEP-induced vertical translation of live cells in conjunction with Particle Image Velocimetry (PIV) in order to measure probabilistic distribution of live cell DE signatures on an entire cell population. The method is integrated in a micro-fluidic device. It is less sensitive to misalignment of microchannel to electrode topologies enabling PDMS soft lithography to be used for the device fabrication. The bottom of the micro-fluidic channel is lined with an Interdigitated (ID) electrode array. Cells passing through the micro-channel are acted on by sedimentation forces, while DEP forces either oppose sedimentation, support sedimentation, or neither, depending on the DE signatures of the cells. The heights at which cells stabilize correspond to their DE signature and are measured indirectly using PIV. The presented method enables simultaneous and high-throughput collection of hundreds of single-cell responses in a single frame. In addition, PIV may be further integrated with fluorescence measurements yielding correlations between DE signatures and intracellular processes.
Dielectrophoretic cell focusing
Dielectrophoretic cell focusing microfluidic device developed at Georgiev Lab. The presence of an electric field tuned to a specific frequency allows the cells to be focused by negative dielectric forces to the center part of the channel.
The media player is loading...