Contents
Experimental Design
Experiments
14.5.2015
1. PCR of streptavidin gBlock
Specs:
- m(tDNA) = 1 ng/reaction
- primers = M13 FWD (-20); M13 FWD (-46)
- cycles = 25
- t(pol) = 50s
- T(gradient) = 50,2°C; 54,6°C; 60,9°C
- polymerase = 95% Taq + 5% Pwo
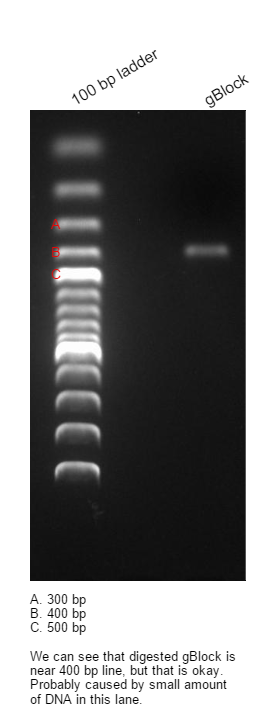
- length(gBlock) = 427 bp
Notes: We should use Phusion polymerase and 12 cycles max in this reaction, but since purification of the resulting product is done twice (20 s exposure to UV), mutations will be present either way. Let's hope some clone will be mutation free.
2. Electrophoresis of PCR product - 2x 1,5%; 0,8%
We ran three gels, first gel showed us that changing annealing temperature in range 50°C-60°C, has no effect on concentration of final PCR product. Second gel(0,8%) was used for purification of the PCR product, purification was done with ROCHE PCR Purification Kit. Concentration of DNA in final solution is 13 ng/ul(Nanodrop). Third gel confirms successful purification of digested gBlock, digestion was done with BamHI and NheI REs.
18.5.2015
1. Transformation of cells with pCT302 plasmid
Transformation of XL-1 Blue cells was done by electroporation(50 ul of cells, 3 ul of DNA). Cells were incubated for 1 hour(37°C) in non-selective TY medium and subsequently on TY-amp plates overnight(37°C).
19.5.2015
1. Preparation for miniprep
Liquid TY-amp medium(3 ml) was inoculated with a mixture of transformed cells and incubated in 37°C o/n.
20.-22.5.2015
1. Miniprep - pCT302 and electrophoresis 1,2%, restriction digest and gel purification
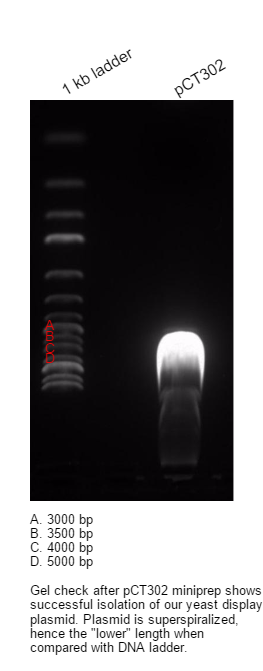
Miniprep was done with Renogen Plasmid Preparation Kit, DNA was eluted with 80 ul of ddH20. Concentration of DNA in soultion was 564 ng/ul(Nanodrop). Plasmid was then digested with BamH and NheI in three separate reactions: 1. only BamHI 2. only NheI 3. BamHI and NheI. Both BamHI and NheI worked OK in 1x Tango buffer, since it was overnight restriction digest, we used only 50%(BamHI) and 25%(NheI) of recommended units for 2 hour digest. There is a ~6000 bp band in every sample, this should be linearized pCT302(BamHI, NheI) and linearized pCT302 without linker(BamHI and NheI), since linkers are usually very short, we cannot see length difference on gel. Subsequent to this analysis, we ran another overnight restriction digest with BamHI and NheI and about 3ug of template DNA - pCT302. Digested plasmid was then purified with Promega Gel Purification Kit and eluted with 40 ul of ddH20, concentration of DNA in soultion was 30 ng/ul.
23.5.2015
1. Ligation of final construct pCT302-Streptavidin(pYDS)
Before ligation, digested pCT302 construct was dephosphorylated with shrimp alkaline phosphatase(to reduce ligation noise). Ligation was done overnight in 16°C, insert to plasmid ration was 3:1.
24.5.2015
1. Transformation with pYDS
Electrocompetent XL-1 Blue cells were transformed directly with the ligation mixture, 70 ul of cells and 5 ul of ligation mixture. Cells were incubated for 1 hour(37°C) in non-selective TY medium and subsequently on TY-amp plates overnight(37°C).
25.5.2015
1. Patches and preparation for clone screening
We made patch plates(TY-amp) with 50 transformants, incubation in 37°C o/n.
26.5.2015
1. STET Minipreparation and restriction digest, gel analysis
Construct(pYDS) for final digest analysis was prepared by STET method. We used 20 selected clones for this screening. Purified plasmids were digested overnight with BamHI and NheI. Analytic gel(1,2%) shows presence of both insert(streptavidin) and plasmid bone(pCT302) in all analyzed clones. A - 250 bp, B - 500 bp, C - 6000 bp
27.5.2015
1. Miniprep - pYDS(3clones), preparation for sequencing and sequencing
Clones C4, C7 and C17 were picked for sequencing. Prior to that, we had to prepare the plasmid DNA(Renogen Plasmid Preparation Kit).